From the busy streets of Toronto to the rural villages of Mozambique, the field is the laboratory for UHN’s Dr. Kevin Kain, pictured in his lab, (L), and with members of an international research team at a hospital in Mozambique. (Photos: UHN StRIDe Team, Courtesy Kevin Kain)
Every parent knows the sinking feeling that accompanies waking to find their child burning up with a fever. Uncertainty and anxiety loom as they grapple with the age-old question: is this a fleeting ailment or a cause for medical attention?
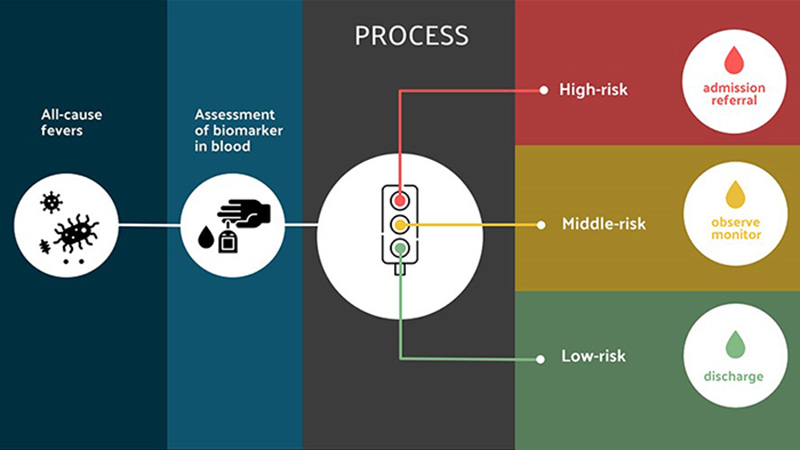
Fortunately, a revolutionary tool is on the horizon – one that, with the simplicity of a finger prick, could empower health care workers, and eventually parents alike, with a reliable means to predict the severity of fever-causing infections.
“Worldwide, approximately two billion cases of fever are recorded each year,” says Dr. Kevin Kain, a Senior Scientist at UHN’s Toronto General Hospital Research Institute and Scientific Director of the Tropical Disease Unit at Toronto General Hospital. “The vast majority of cases are mild and resolve on their own, but one or two per cent progress to severe or even fatal illnesses.”
The problem is that there are no good tools to identify the small group of unlucky people for whom a fever is a harbinger of life-threatening disease. This leaves us overtreating with antibiotics, worsening the global crisis of antimicrobial resistance, and overadmitting to hospitals, increasing the burden on already strained health care systems.
Beyond providing no benefit to most patients, our current approach may also cause harm by subjecting them to unnecessary diagnostic tests and treatments and putting them at risk of serious hospital-acquired infections.
“The key is to be able to determine who is at risk of severe and fatal disease, early in the course of their illness,” explains Dr. Kain, who is also Director of the Sandra A. Rotman Labs at the Sandra Rotman Centre for Global Health and Professor of Medicine at the University of Toronto. “This will allow us to identify which patients need to be admitted and aggressively managed, versus the majority who would be better off recovering at home.”
This is a particularly difficult task in regions of the world that have limited diagnostic tools and health care resources, such as sub-Saharan Africa. There, young children typically experience upwards of six episodes of fever every year, and up to 70 per cent of severe infections are missed, leading to high mortality rates.
To address this issue, Dr. Kain and his collaborators have developed an innovative finger-prick blood test that can help to predict a patient’s risk of severe outcomes, early in their illness, regardless of the type of infection they have.
Now, the research team is evaluating the test in the field through an international consortium of 13 academic and industry partners from North America, Europe and Africa, called EChiLiBRiST.
A far-fetched idea
According to Dr. Kain, the world-spanning research project was born from a seemingly far-fetched idea: what if the microbe itself is not all that important for predicting patient outcomes?
Vastly different infections, from malaria to COVID-19, behave in similar ways. Many people get infected, but only a small proportion become critically ill. Among those who do, common physiological processes lead to serious complications or death, and these processes are accompanied by distinct molecular features in the blood.
“As a clinician, you often see the same infection play out very differently in different patients,” says Dr. Kain. “Usually, most people recover quickly and come through unscathed, but a few end up with sepsis and long-term complications.
“It all comes down to their response to the infection.”
When the immune system is overactivated by bacteria, viruses or other invading organisms, it releases inflammatory molecules that alter the stability of endothelial cells – the cells that line blood vessels. This process – called endothelial activation – weakens the vessels and causes them to become leaky, eventually leading to multi-organ damage, a process called sepsis.
In a series of studies conducted over the past 10 years, Dr. Kain and his team have discovered circulating proteins that signal vessel damage in people with malaria, pneumonia, dengue, COVID-19 and other causes of sepsis. Across diseases, high levels of these proteins serve as early warning signs that a person is likely to experience sepsis.
The team’s findings have been published in numerous journals, including Clinical Infectious Diseases, Nature Communications and PLoS Medicine.
These studies, which took place in Uganda, Tanzania, Mozambique and several other countries, laid a strong foundation for developing a blood test that measures markers of endothelial activation to predict a patient’s risk of becoming seriously ill.
A hard sell
“We got a lot of push back initially, to put it mildly!” says Dr. Kain.
“Most infectious disease researchers are focused on microorganisms, rather than the host response, so the idea of shifting the spotlight to immune and endothelial activation was a hard sell.”
The group’s initial funder for the research was the Defense Advanced Research Projects Agency (DARPA) – the research agency of the United States Department of Defense.
The military was interested in determining whether Dr. Kain’s approach could be used to address the threat of synthetic bioweapons.
“The fear at the time was that someone could insert genes for lethal microbial toxins into the genomes of bacteria that normally colonize us and do not make us sick, like a Trojan horse,” Dr. Kain explains. “If they set those altered bacteria loose in a public setting, our traditional diagnostics would be useless at detecting who was exposed to the toxin because we wouldn’t know what bug we’re looking for.
“They liked the idea of being able to test if someone had been exposed to a toxin without knowing which bacteria was responsible, just by looking for markers of endothelial activation.”
Despite the potential military applications of his research, Dr. Kain’s steadfast focus was helping to manage globally important infections, particularly those that afflict neglected populations in low-resource countries.
Fortunately, more groups eventually came around to the idea and the team secured funding from the Canadian Institutes of Health Research, the Tesari Foundation and other funding agencies to continue developing their biomarker screening strategy.

From UHN to Africa
With a recent grant of 8 million euros – more than $11.5 million – from Horizon Europe’s Research and Innovation Program, the EChiLiBRiST consortium is now evaluating the test in two multinational clinical trials in Africa.
“This five-year research project, which is being led by my long-time collaborator, Dr. Quique Bassat, is a major step forward for translating our research into a transformative clinical tool,” says Dr. Kain.
The first trial, taking place in Mozambique and Gabon, will determine whether the biomarker test can improve current triaging strategies for risk-stratifying patients. The second trial, in Mozambique and Ethiopia, will assess whether the biomarkers can also serve as targets for therapy in severely ill febrile children.
The consortium is also working with the biotech company BioEclosion to determine the cost-effectiveness, scalability and cultural acceptability of the technology. These studies are laying the foundation for translating the test into an easy-to-use, rapid and inexpensive point-of-care tool.
“It is really gratifying to see this test – which arose largely from research conducted at UHN and funded by Canadian sources – be evaluated in populations that have so much to gain from it,” says Dr. Bassat, an ICREA Research Professor at the Barcelona Institute of Global Health (ISGlobal) and a principal investigator for the EChiLiBRiST project.
Although the initial clinical trial sites are in Africa, Dr. Kain emphasizes that the test could have major health and economic impacts worldwide.
“As a general rule, if a clinical tool works in low-resource settings like this – it will work anywhere,” he says.
The test could be a game changer everywhere from the crowded Emergency Department at UHN to remote clinics serving Indigenous communities. Eventually, the test could also be available to the public for home use, like rapid COVID tests.
From field side to bench
Dr. Kain and his team rarely follow the traditional “bench to bedside” approach to research. Instead, they describe their unique workflow as “field side to bench, and back to field side.”
“If you think a physiological process is important in a particular condition, first show a clear epidemiological link in real people with real disease, then confirm causality in preclinical studies and develop a test or intervention in the lab,” explains Dr. Kain.
“Once you’ve done that, you can go back to the field and evaluate your test or intervention in clinical trials in settings that have high disease burden. This is what we are doing now with EChiLiBRiST – closing the translational loop.”
After more than 15 years of research and international collaborations, Dr. Kain’s innovative test has the potential to transform the way we manage infections globally – reducing deaths, disability and wasted health care dollars.
And hopefully, one day, helping parents sleep a little easier when a fever strikes.
This study was supported by generous donors to UHN Foundation.