A research team at UHN’s Donald K. Johnson Eye Institute, in collaboration with the Institute of Biomedical Engineering at the University of Toronto (U of T), has discovered that transplanted retinal cells can share essential materials with host cells in the lab, offering a promising avenue for delivering therapies directly to damaged areas of the eye.
The degeneration of photoreceptors, a leading cause of blindness affecting one in 2000 individuals globally, stems from the loss of light-sensing cells in the eyes. It is an irreversible condition due to the limited regenerative capacity of our central nervous system.
Cell therapy has been explored as a potential vision restoration treatment, involving the introduction of healthy donor cells into the retina to form new connections with surrounding neurons.
However, studies have shown that transplanted cells rarely form new connections. Instead, they transfer cellular materials, such as proteins, into the host retina in a process called material transfer.
“This bidirectional process has been observed mainly in experimental models,” explains Dr. Margaret Ho, a Vanier scholar, a PhD candidate at the Institute of Biomedical Engineering at U of T and first author of this eye study.
“However, until now, it has remained unclear whether material transfer can occur between human cells.”
“We want to understand if human photoreceptors derived from stem cells can engage in material transfer, as this could be a promising pathway for targeted drug delivery to diseased cells,” explains Dr. Valerie Wallace, Senior Scientist at UHN’s Krembil Research Institute and a senior author of this study.
This collaborative project draws on the complementary expertise of Dr. Wallace and Dr. Molly Shoichet. Dr. Wallace specializes in material transfer, experimental models and tissue analysis, while Dr. Shoichet focuses on in-human stem cell growth and manipulation, and the creation of photoreceptors from retina tissue-like structures called retinal organoids.
“This work couldn’t have been possible without the collaboration between our labs,” said Dr. Shoichet, a professor at the U of T and one of the corresponding authors of the research.
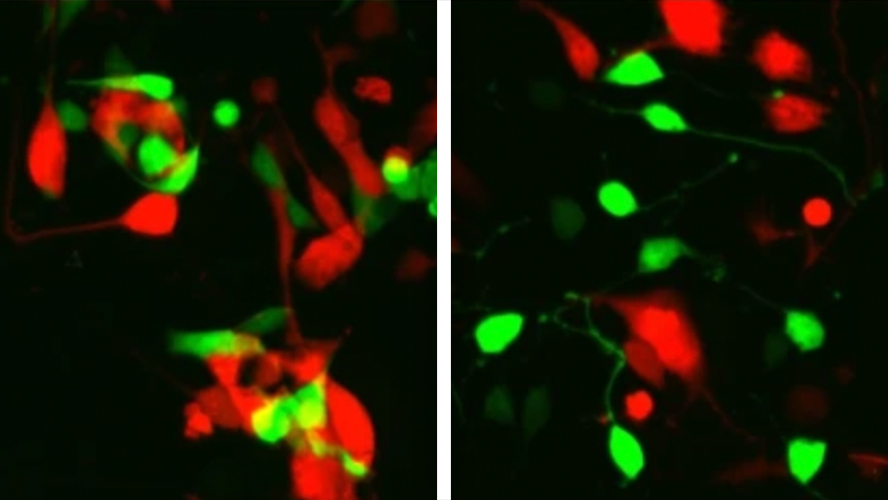
The researchers used two approaches to understand if human stem-cell-derived photoreceptors can transfer materials to host cells. First, they tested this by transplanting their human donor photoreceptors into experimental models with intact host photoreceptors, and then they tested species specific transfer by co-culturing donor and host cells from dissociated human retinal organoids in a lab dish.
In the first set of experiments, they tried transplanting human photoreceptors from different stages of development into experimental models with eye problems. No transfer was observed, even though both cell types survived in the eye the same amount of time.
In the second experiment in the lab, researchers observed that human donor cells could transfer their content to host cells, which has never been shown before.
This phenomenon opens up an entirely new way of thinking about vision repair.
“This result suggests the importance of considering species when designing models to study material transfer in the future,” emphasizes Dr. Ho.
“Photoreceptors derived from human stem cells could still hold promise for treating eye diseases in humans,” says Dr. Wallace. “We need to keep studying how to optimize cell transplants to advance treatments for eye disease and improve transplant outcomes.”
These findings are a step forward in our understanding of transplant therapy for eye disease. This advancement prompts new questions about whether human eye cells possess the same capabilities, an area largely unexplored due to prior studies focusing on late-stage eye disease models devoid of host cells.
This study was supported by generous donors to UHN Foundation.