At UHN’s McEwen Stem Cell Institute, researchers are building on these advances and working tirelessly to develop innovative cells to treat diseases with the most critical need. They are collaborating with clinicians at UHN to make these treatments a reality and significantly improve the lives of patients.
Here are some of the groundbreaking things happening at UHN’s McEwen Stem Cell Institute.
Regenerating heart tissue after a heart attack
Heart attacks damage the heart muscle. As the heart heals, that damaged muscle is replaced with scar tissue that stiffens the heart and limits its ability to beat, putting stress on the heart and resulting in changes that can lead to heart failure. Fifty per cent of those diagnosed with heart failure will die in the first five years after their diagnosis and there is currently no treatment available to patients to reverse this damage.
Dr. Michael Laflamme is using stem cells to regenerate healthy heart muscle following a heart attack. He has made heart cells from these stem cells that, when injected into the damaged region of the heart, can replace the scar tissue with healthy, functional heart muscle. This treatment could lead to monumental improvements in the quality of life for patients following a heart attack.
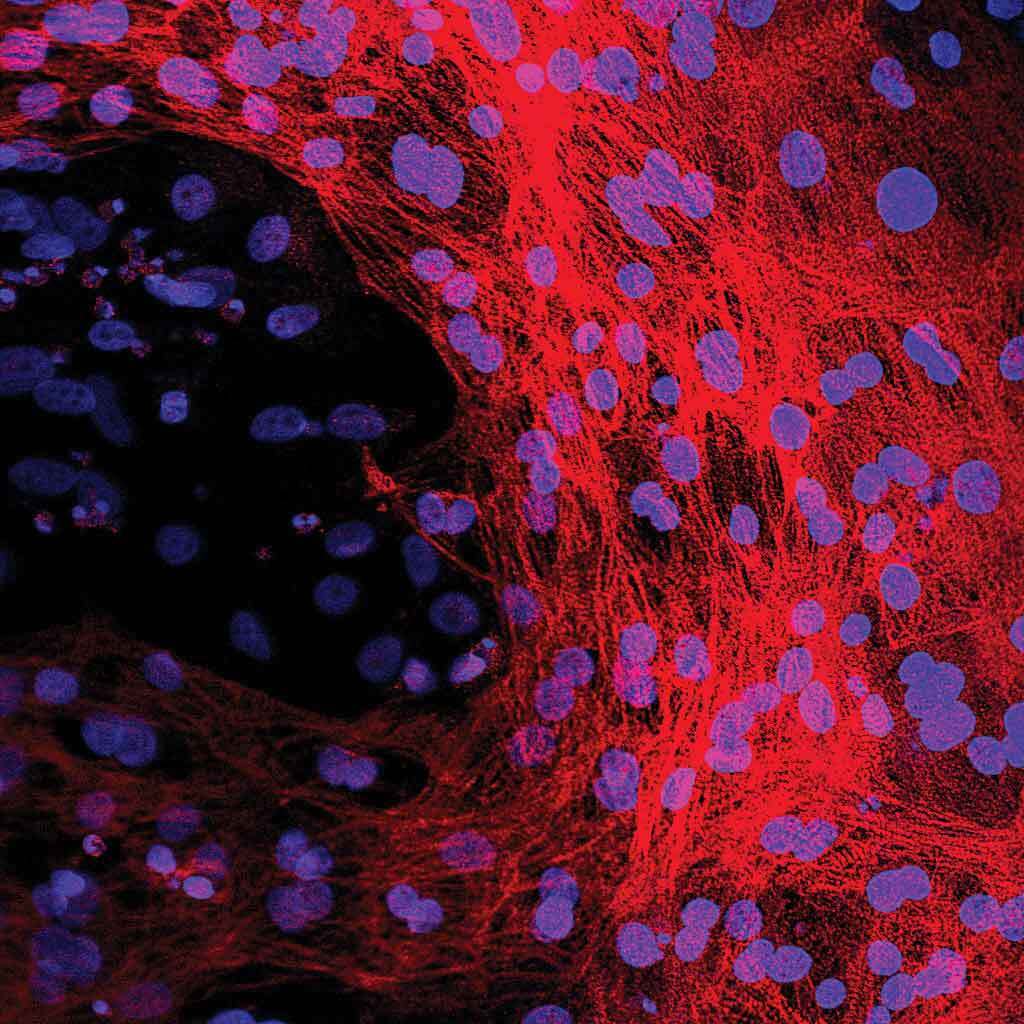
Microscopic image of heart muscle cells made from stem cells. The blue stain shows the location of each heart muscle cell’s DNA while the red stain shows the rest of the heart muscle cell and the complete structure they are forming.
Creating insulin-producing cells to treat diabetes
Type 1 diabetes is a chronic condition affecting more than 300,000 Canadians where the cells in the pancreas are unable to produce insulin, a vital hormone needed to regulate blood sugar in the body. Individuals with this condition need to administer insulin regularly to maintain their blood sugar levels. Uncontrolled blood sugar can lead to life-threatening complications including kidney, heart, nerve and eye damage.
Dr. Cristina Nostro is creating insulin-producing cells from stem cells that, based on preclinical studies, have the potential to restore normal blood sugar regulation. If these insulin-producing cells show similar promise in clinical trials, this cell therapy approach could dramatically change the lives of patients, eliminating the need for blood sugar monitoring and insulin injections.
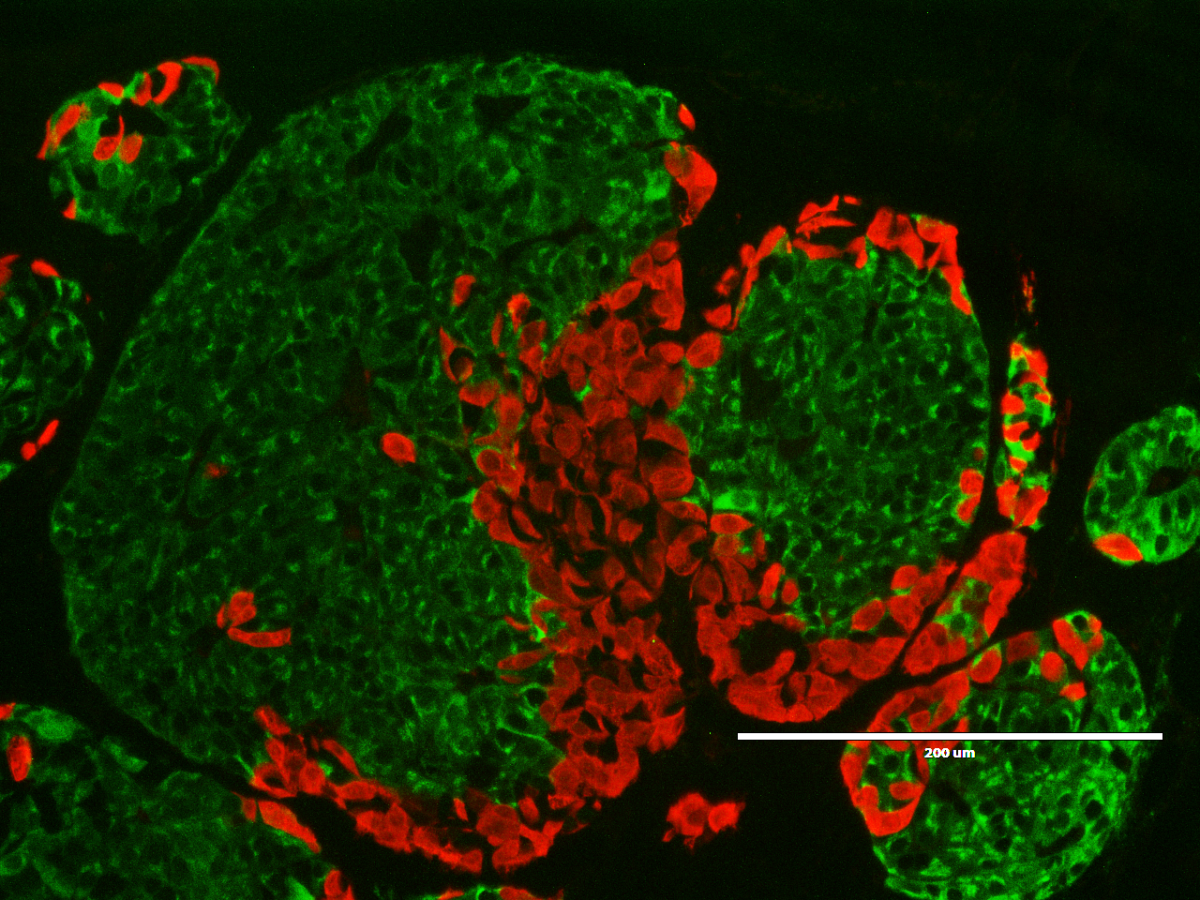
Microscopic image of insulin-producing cells made from stem cells. Each green dot is a cell that is producing insulin.
Repairing damaged liver tissue
For those with end-stage liver failure, a liver transplant is the only curative treatment option. However, there are not enough donor organs available and as a result, these transplants are difficult to obtain. In 2018, there were 424 individuals on the waiting list for liver transplants in Canada, 76 of whom died while waiting for a liver.
Dr. Shinichiro Ogawa is developing liver cells from stem cells for the treatment of end-stage liver failure. The goal is to transplant these cells into damaged livers to regenerate healthy, functional tissue. If successful, this cell therapy could restore normal liver function, eliminating the need for a transplant.
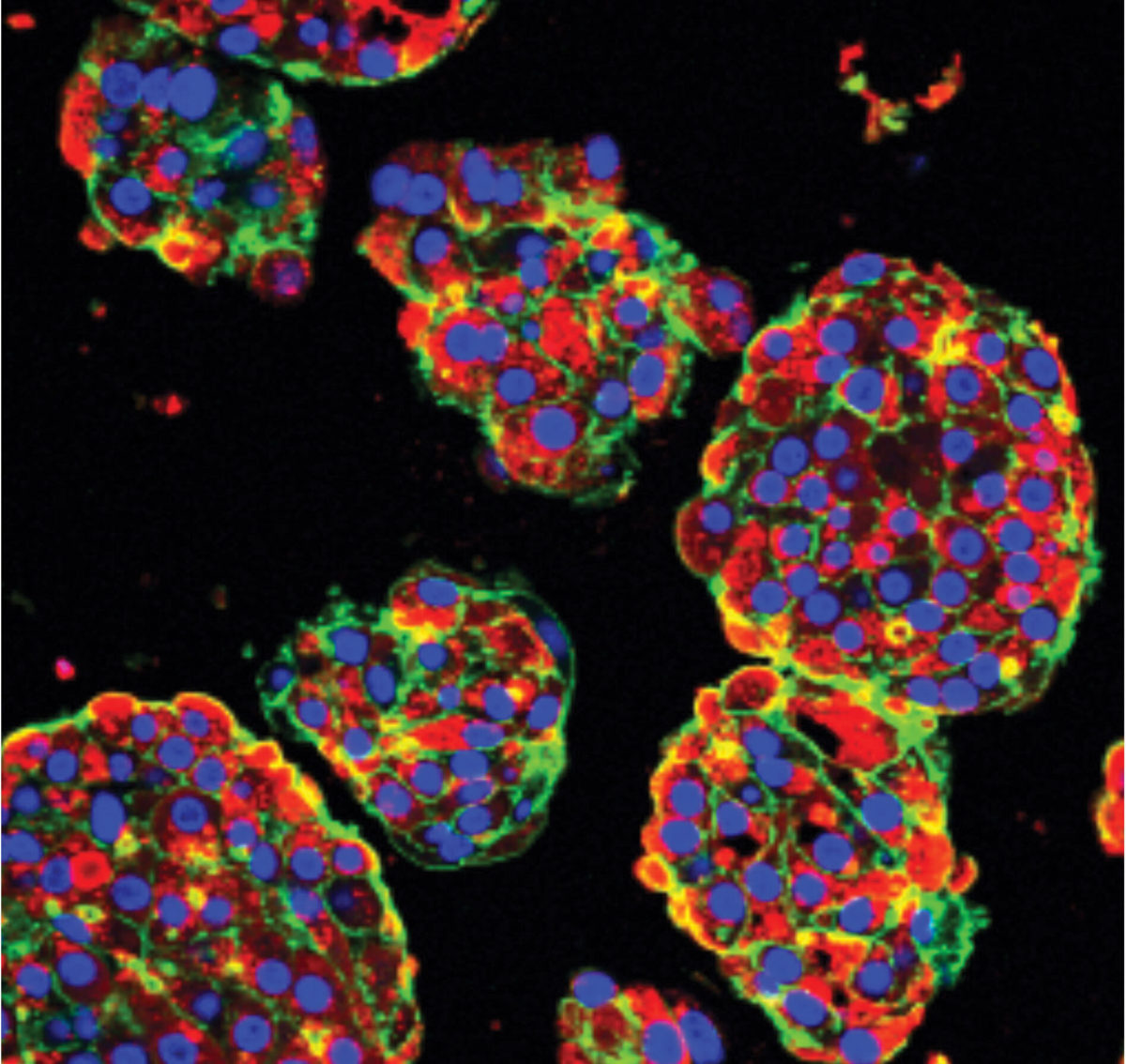
Microscopic image of human liver cells made from stem cells. Each blue dot represents a separate liver cell.
Building a biological pacemaker
Located in the heart, pacemaker cells use electrical impulses to set the rate and rhythm at which the heart pumps blood. In some individuals, these pacemaker cells become dysfunctional or dysregulated, leading to an irregular heartbeat. Treatment for this condition involves implanting an electronic pacemaker, which uses electrical signals to override the pacemaker cells and restore normal heart rhythm and rate. While these pacemakers have been highly successful, their batteries must be replaced every five to ten years through invasive surgery, which can lead to severe infections and other complications.
Dr. Stephanie Protze is building the first-ever biological pacemaker from stem cells. She has developed heart pacemaker cells from these stem cells that can regulate the rate and rhythm at which heart muscle cells contract. Unlike electronic pacemakers, these cells should be able to respond to hormonal signals, increasing heart rate in response to stressors. Biological pacemakers have the potential to profoundly improve the quality of life for patients with pacemaker cell disorders.
Generating transplantable blood cells
Blood-forming stem cells found in the bone marrow are responsible for producing the different types of blood cells throughout life. Diseases in the blood-forming system, such as leukemia, disrupt this process and can progress to life-threatening conditions in which an inappropriate number of blood cells are produced. Treatment and eradication of leukemia often destroy the bone marrow’s ability to produce blood. A bone marrow transplant can provide patients with the stem cells needed to regenerate a healthy and fully functioning blood-forming system. While this treatment is effective, bone marrow donors are limited.
Dr. Gordon Keller is working to make all the different types of blood cells, including those formed from stem cells located in the bone marrow. The ability to make blood-forming cells would provide a new source of these types of cells for transplantation to treat blood cell disorders.
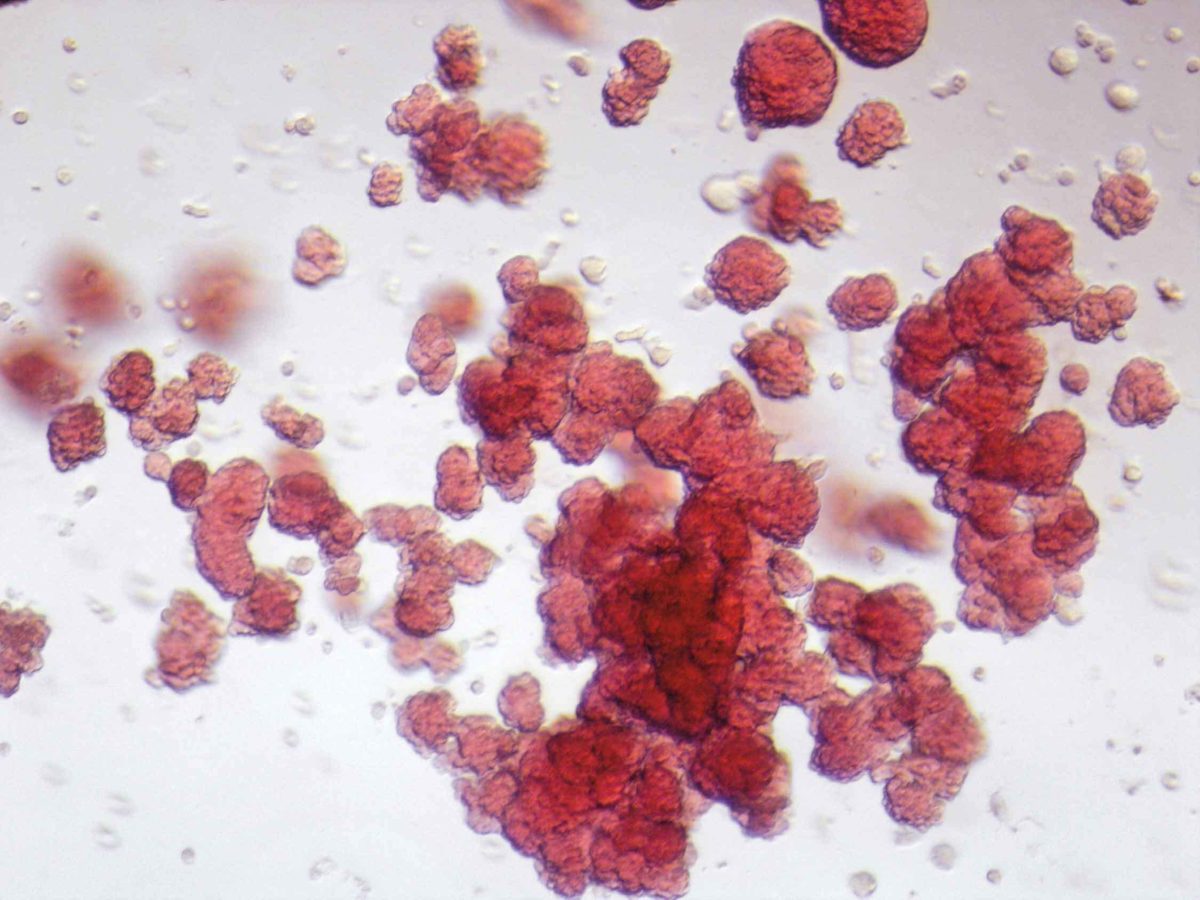
Microscopic image of blood cells made from stem cells. Each red sphere is a separate blood cell.
With generous donor support, the world-leading team at University Health Network’s McEwen Stem Cell Institute is developing groundbreaking treatments for those in greatest need. Thank you for your continued support and for helping to bring life-changing treatments to the patients who need them most.